WVU Physicists Give The First Law Of Thermodynamics A Makeover
Eddie Gonzales Jr. – MessageToEagle.com – West Virginia University physicists have made a breakthrough on an age-old limitation of the first law of thermodynamics.
Paul Cassak, professor and associate director of the Center for KINETIC Plasma Physics, and graduate research assistant Hasan Barbhuiya, both in the Department of Physics and Astronomy, are studying how energy gets converted in superheated plasmas in space.
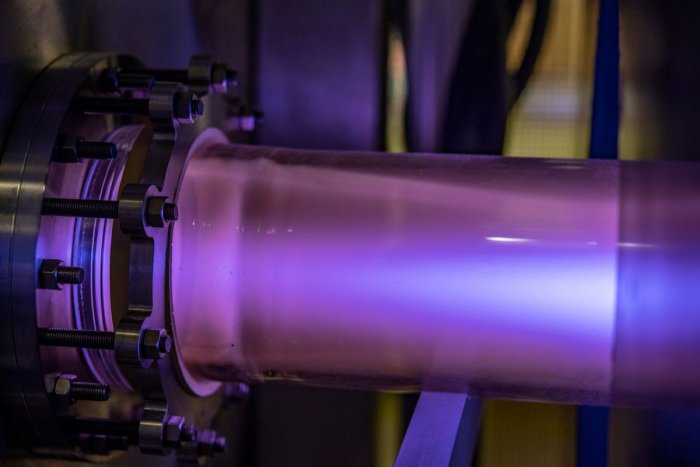
In this photo, argon plasma glows a bluish color in a Center experiment. Image credit: WVU /Brian Persinger)
Their findings, funded by a grant from the National Science Foundation and published in the Physical Review Letters journal, will revamp scientists’ understanding of how plasmas in space and laboratories get heated up, and may have a wide variety of further applications across physics and other sciences.
The first law of thermodynamics states that energy can neither be created nor destroyed, but it can be converted into different forms.
“Suppose you heat up a balloon,” Cassak said in a press release.
“The first law of thermodynamics tells you how much the balloon expands and how much hotter the gas inside the balloon gets. The key is that the total amount of energy causing the balloon to expand and the gas to get hotter is the same as the amount of heat you put into the balloon. The first law has been used to describe many things — including how refrigerators and car engines work. It’s one of the pillars of physics.”
Developed in the 1850s, the first law of thermodynamics is only valid for systems in which a temperature can be properly defined, a state known as equilibrium. As an example, when combined, a cup of cold water and a cup of hot water will eventually reach a warm temperature between them. This warm temperature is the equilibrium. However, when the hot and cold water have not yet reached that endpoint, the water is out of equilibrium.
Likewise, in many areas of modern science, systems are not in equilibrium. For over 100 years, researchers have attempted to expand the first law for common materials not in equilibrium, but such theories only work when the system is nearly there — when the hot and cold water are almost mixed. The theories do not work, for example, in space plasmas, which are far from equilibrium.
The work of Cassak and Barbhuiya fills in the blanks on this limitation.
“We generalized the first law of thermodynamics for systems that are not in equilibrium,” Cassak said. “We did a pencil and paper calculation to find how much energy is associated with matter not being in equilibrium, and it works whether the system is close to or far from equilibrium.”
Their research has numerous potential applications. The theory will help scientists understand plasmas in space, which is important for preparing for space weather. Space weather occurs when huge eruptions in the solar atmosphere blast superheated plasma into space. It can cause problems like power outages, interruptions to satellite communications and the rerouting of airplanes.
“The result represents a really large step of our understanding,” Cassak said. “Until now, the state-of-the-art in our research area was to account for energy conversion only associated with expansion and heating, but our theory provides a way to calculate all the energy from not being in equilibrium.”
“Because the first law of thermodynamics is so widely used,” Barbhuiya said, “it is our hope that scientists in a wide array of fields could use our result.”
For example, it may be useful for studying low-temperature plasmas — which are important for etching in the semiconductor and circuit industry — as well as in other areas like chemistry and quantum computing. It might also help astronomers study how galaxies evolve in time.
Groundbreaking research related to Cassak and Barbhuiya’s is being carried out in PHASMA, the PHAse Space MApping experiment, in the WVU Center for KINetic Experimental, Theoretical and Integrated Computational Plasma Physics.
“PHASMA is making space-relevant measurements of energy conversion in plasmas that are not in equilibrium. These measurements are totally unique worldwide,” Cassak said.
Likewise, the breakthrough he and Barbhuiya have made will change the landscape of plasma and space physics, a feat that doesn’t happen often.
“There aren’t many laws of physics — Newton’s laws, the laws of electricity and magnetism, the three laws of thermodynamics, and the laws of quantum mechanics,” said Duncan Lorimer, professor and interim chair of the Department of Physics and Astronomy. “To take one of these laws that has been around over 150 years and improve on it is a major achievement.”
“This new first principles result in non-equilibrium statistical mechanics as applied to plasmas is a great example of the academic research enabled by NSF’s mission ‘to promote the progress of science’,” said Vyacheslav Lukin, a program director for plasma physics in the NSF Division of Physics.
Written by Eddie Gonzales Jr. – MessageToEagle.com Staff